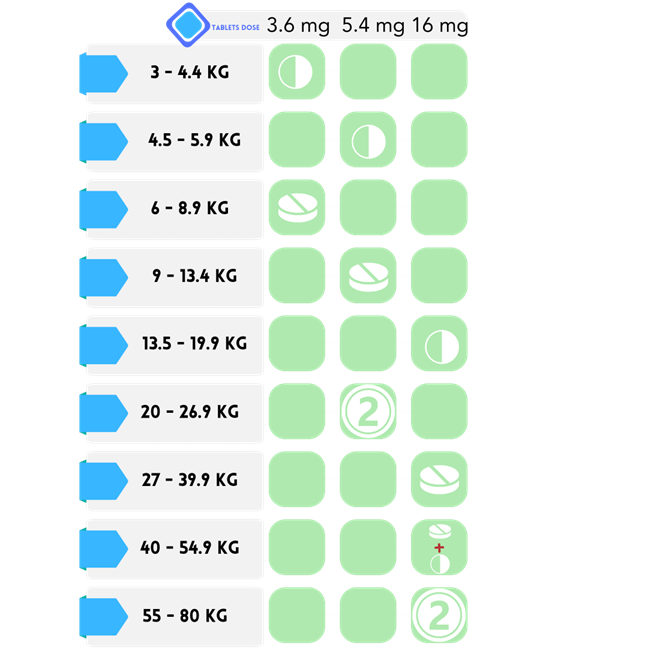
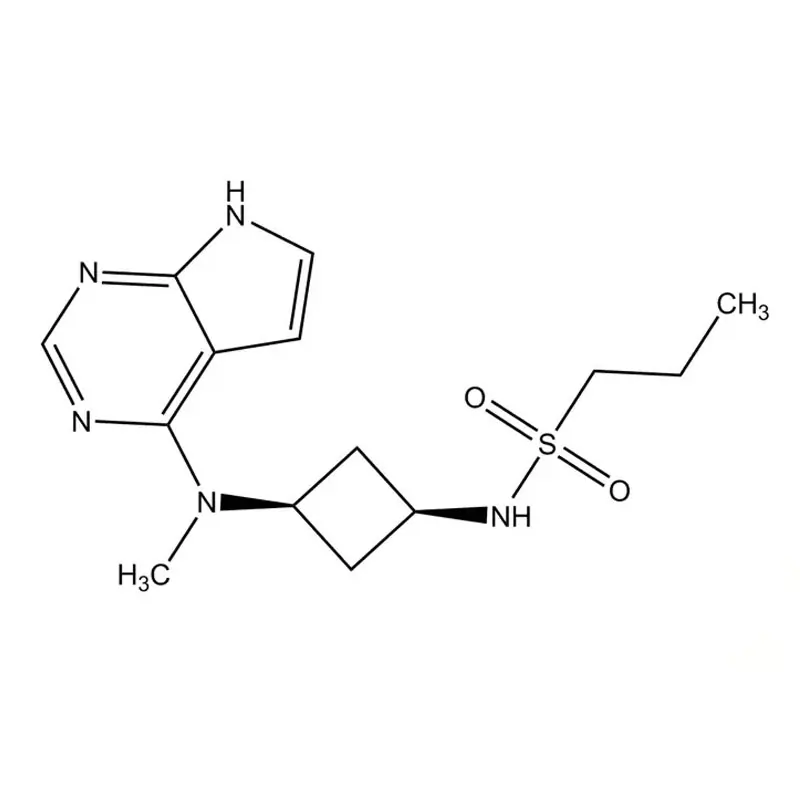
Apocitinib : OCLACITINIB MALEATE 3.6, 5.4, 16 mg Tablets
Mechanism of action:
While not being classified as an antihistamine, Oclacitinib functions by suppressing the activity of numerous pruritogenic cytokines and pro-inflammatory cytokines, along with cytokines associated with allergic reactions that rely on the enzymatic activity of JAK1 or JAK3. However, it exerts minimal impact on cytokines involved in hematopoiesis that rely on JAK2.
Apocitinib is a veterinary medication that helps alleviate itching and inflammation in dogs. This condition can cause discomfort for our beloved pets, leading to rashes, scratching, and licking. It is commonly prescribed for allergic dermatitis and atopic dermatitis. Apocitinib targets specific enzymes involved in the itching and inflammation process, providing relief for dogs with these conditions. However, it is crucial to follow veterinary guidance and dosage recommendations when administering Apocitinib, as it may have side effects and interactions that require monitoring.
Indications: Pruritus management in dogs with allergic dermatitis and control of atopic dermatitis in dogs aged 12 months or older.
How To Use: Tablets are suitable for consumption with or without food.
Required Considerations: It is important to wash your hands promptly after handling them. Ensure that the product is stored at a controlled room temperature, specifically between 20-25 degrees Celsius(68-77F). To prevent accidental overdose, keep the medication out of reach of pets. WARNINGS: Apocitinib should not be administered to dogs under the age of 12 months. Additionally, it should not be used in dogs with severe infections. It is important to note that Apocitinib may heighten the risk of infection, such as demodicosis, and worsen neoplastic conditions.
Pharmacokinetic:
Dogs absorb Oclacitinib maleate quickly and effectively when taken orally, reaching peak plasma concentrations in less than 1 hour. When 0.4-0.6 mg Oclacitinib/kg was given orally to 24 dogs, the maximum concentration in the blood was 324 ng/mL (with 90% confidence limits of 281 and 372 ng/mL), and the area under the plasma concentration-time curve was 1890 ng∙hr/mL (with 90% confidence limits of 1690 and 2110 ng∙hr/mL). The prandial state of the dogs does not affect the absorption rate or extent. The absolute bioavailability of Oclacitinib maleate is 89%. Oclacitinib has low protein binding, with 66.3-69.7% bound in fortified canine plasma at concentrations ranging from 10-1000 ng/mL. The apparent volume of distribution at steady-state is 942 mL/kg body weight (with 95% confidence limits of 870 and 1014 mL/kg). In dogs, Oclacitinib is metabolized into multiple metabolites, and one major oxidative metabolite was found in the plasma and urine. The main clearance route is metabolism, with minor contributions from renal and biliary elimination. Oclacitinib has minimal inhibition on canine cytochrome P450 enzymes, with inhibitory concentrations 50 times greater than the observed concentrations at the recommended dose. The total body clearance of Oclacitinib from plasma is low, with a mean value of 316 mL/h/kg body weight (with 95% confidence limits of 237 and 396 mL/h/kg body weight, or 5.3 mL/min/kg body weight). The terminal elimination half-life after intravenous and oral administration is similar, with mean values of 3.5 hours (with 95% confidence limits of 2.2 and 4.7 hours) and 4.1 hours (with 95% confidence limits of 3.1 and 5.2 hours), respectively.






Reviews
There are no reviews yet.